Objectives:
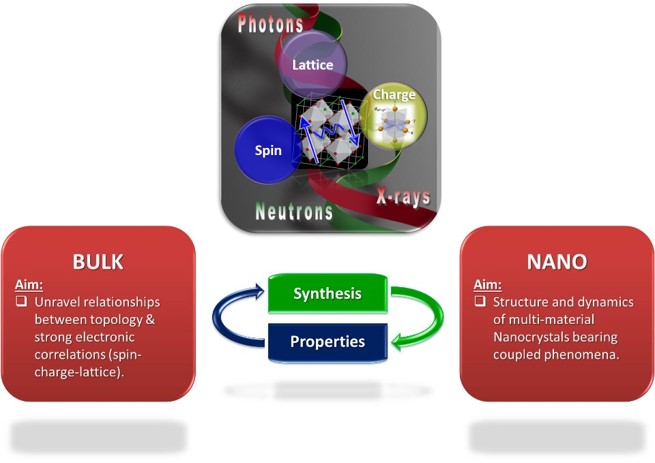
The lab studies quantum materials, an umbrella of systems engaging atomic-scale building blocks, bearing strongly interacting elementary units (cf. charge carriers and electric/ magnetic moments) that give rise to collective phenomena, with remarkable physics (cf. quantum size-effects, spin liquids, superconductors, magnetoelectrics etc).
From size-controlled hybrid nanorystals to molecule-based materials, atomic structure insights enable the exploitation of selected crystalline solids for opportunities in innovative theranostics (e.g. image-guided therapeutics) or novel ways to supersede limitations in energy technologies (e.g. power transmission & use).
With the purpose to understand how the material's atoms are arranged and how their elementary units function, team members are encouraged to exploit our facilities in order to
- prepare/create solid-state compounds, for example, pertinent to mixed-valent transition metal oxides (chalcogenides or halides)
- measure basic physical properties, such as magnetic susceptibility, dielectric permittivity and electrical/thermal transport or optical activity
- correlate the macroscopic physical properties with the nanoscale rearrangements observed by synhcrotron X-ray and neutron scattering methods
- develop experimental tools involving external stimuli (e.g. pressure, magnetic field, laser light etc).
More elaborate experiments are done in collaboration with other research groups around the world. Strong links with theoretical teams assist our experimental efforts to choose the optimum set of materials and to justify our experimental findings.
Research Topics
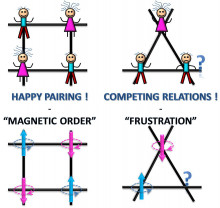
Motivation:
Frustration arises when a system cannot minimize all the pair-wise interactions simultaneously because of local geometric constraints. Competing or frustrated interactions extend beyond the condensed matter physics and into biological materials, as for example, nature has the ability to "resolve" frustrated interactions in order to carry out some targeted biological activity.
Activity:
Frustration may give-rise to novel and complex phenomena that motivate us to (i) develop new class of materials, (ii) study cooperative phenomena in magnetism (e.g. magnetoelectric coupling) that provide fertile ground for testing theories of interacting systems that possess different spatial dimensions and sign of interactions that may impose local anisotropy of the basic interacting unit, the spin. Such fundamental studies can uncover mechanisms that benefit potential applications in low-power consumption devices and/or energy havesting technologies.
Literature:
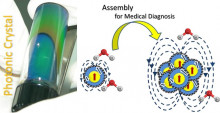
Motivation:
Nanostructures with controlled size and shape, where magnetic and optical properties coexist, are most promising for innovative technologies (e.g. ranging from information storage to biomedical theranostics). Such a demand may be well-addressed when enhanced or collective magnetic/ photonic properties are established in nanoscale systems made of multiple subunits arranged in a controlled topological fashion through heteroepitaxial connections or self-assembled in secondary (e.g. cluster-like) structures.
Activity:
A major focus of our team entails the combination of chemistry and physics for the development of inorganic nanomaterials with potential in diverse applications. For this purpose, elaborate colloidal chemistry strategies are employed to control the nucleation and growth of nanocrystals (cf. zinc-blend, rock-salt, spinel, perovskite structural types) at moderate temperatures, while their multifunctional character is studied by bulk and local probe techniques. Moreover, directed-assembly of inorganic nanocrystals is chosen to provide a facile avenue to impart a collective nature in application-specific physical properties, offering the possibility for magnetically-driven image-guided diagnosis and therapy.
Literature:
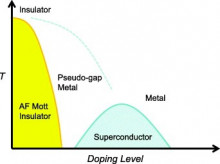
Motivation:
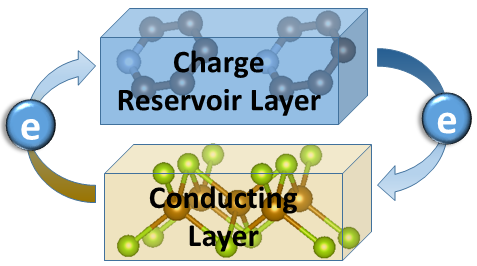
Limitations of energy materials, and in particular for power transmission and storage, necessitates to decipher their workings at different length and time scales. In such an endeavor, cutting-edge tools are developed and implemented in real-word environments. Unique insights drawn in this way at the molecular or atomic level of matter, help understanding complex phenomena that are fundamentally important for discovering smart energy solutions. Amongst others, materials that could potentially transport electricity with zero resistance (cf. superconductivity) are the holy grail for improving dramatically the energy efficiency in electronics and power applications.
Activity:
It is our aim to identify structural rearrangements that accompany the different flow of electrons (i.e. from the charge reservoir to the conducting layer and vice-versa) in superconductors and the motion of charges in structurally related solid-state battery materials. Our activities devise alternative chemical pathways for single-phase model systems and based on them, attempt to parameterize the conditions leading to phases operating at elevated (critical) temperatures and/or offering the possibility to store higher energy density. The Lab strives to understand the physical or chemical interactions occurring on the atomic scale of intercalated layered transition metal chalcogenides / oxides. The outcomes contribute to clarifying the broader role of electronically-driven locally broken symmetries in triggering a phenomenon and creating useful physical properties.
Literature:
Heads
Technical Staff
Alumni

We are exploring the interface between chemistry and physics of novel correlated electron systems and other bulk or nanoscale quantum materials that tackle energy-efficient technologies.
Activity:
In order to study different aspects of the structure, dynamics and functionality of new materials, the team engages:
- preparative solid-state chemistry & nanochemistry lab portfolios;
- various synthetic techniques including sol-gel and redox precipitation processes, in addition to conventional solid-state and colloidal chemistry high-temperature methods are been implemented. Nanocrystal functionalisation and bulk sample growth optimisations, with tools working at elevated temperatures (up to 1850 K), are available. There is great expertise in handling air-and moisture- sensitive compounds under anaerobic conditions, involving Schlenk lines and dry-box techniques.
- experimental stations for automated physical property measurements;
- materials bearing electronic phenomena and phase transitions, are tackled by a multimodal approach requiring strategic collaborations and appropriate development of in-house magnetic, dielectric, transport experimental and spectroscopic probes that are integrated in sophisticated sample environments, where applied electromagnetic stimuli can be flexibly utilized (e.g. low temperatures, high magnetic fields and elevated pressures).
- unique experimental tools (sought through peer-reviewed proposals) at European (ISIS-UK; PSI-Switzerland; ESRF-FR) and US (SNS-ORNL, NSLS-BNL) user-facilities of neutron, synchrotron X-ray and muon science.
Infrastructure Equipment

DC Magnetometer & AC Susceptometer, based on a MagLab-EXA 2000 multi-measurement system (Oxford Instruments) offers high resolving power in deciphering static and dynamic phenomena in low-moment, correlated electron systems of either bulk or nanoscale forms.
Applications:
Invaluable insights on the physical property materials response by measuring the moment versus applied magnetic field (e.g. hysteresis loops) or moment versus temperature, for deciphering static and dynamic phenomena associated with magnetic and superconducting materials. Its sensitivity is very high, therefore a small amount of sample is enough to ensure reliable signals. Automated measurements allow precise determination of the charasteristics of the superconducting state (e.g. crticial temperature, Meissner effect etc), as well as those of the Curie, Néel, and spin-glass states, including parameters such as the blocking or freezing temperatures of ferromagnetic, antiferromagnetic, superparamagnetic systems.
Specifications:
(a) High magnetic field (H= 0-7 Tesla), (b) Low-temperature liquid Helium cryostat (T= 1.8-350 K), (c) DC moment extraction (~10-4 emu), (d) AC susceptibility (~10-6 emu), f= 0.01-10 kHz, (e) samples of a few milligrams, can be accomodated in diverse morphologies, ranging from films and (nano)crystals to bulk forms – typical container, gelatin capsule ~15 mm long, with O.D. ~5 mm. The software measurement sequencer (open source) provides a set of high level actions to enable you to write and control measurements in a way that suit your own specific requirements.

The facility provides a modular experimental station to study the electrical characteristics of energy materials (e.g. electrode materials for Li/Na-ion rechargeble batteries), and beyond that the evolution of electric and magnetic dipole orders, as well as their degree of coupling, which is an identifying feature of a novel magneto-electric systems (e.g. sensors, high-capacity four-state logic memories etc.).
Applications:
This experimental station provides computer-controlled physical property measurements entailing a home-built modular sample environment offering to probe temperature (down to 2 K) and frequency (up to 2 MHz) dependent phenomena under externally applied electromagnetic stimuli (e.g. magentic fields up to 7 Tesla). It is equipped with custom-designed probes for mounting samples in various forms (e.g. polycrystalline pellet, single crystal, films), while the flexible integration of various types of digital measurement instruments allows automated data collection of physical quantities, including, Capacitance and Dielectric Loss, Voltage, Electric DC Current and Impedance etc.
Specifications:
Indicative capabilities include:
- low level sensitive measurements of current down to 10 aA (10 x10-18 A), electric polarization to charge levels down to 1 fC, very high resistance up to 210 PΩ (1018 Ω) and even I-V characteristics by a two-electrode configuration
- low noise voltage (down to 50 nV) measurements, characterization of low resistance/resistivity specimen by a standard four-wire setup (10 μΩ - 10 MΩ) and even Hall effects by Van der Pauw wiring
- impedance spectroscopy for precise measurement of capacitance and loss over a choice of frequencies, ranging from 50 Hz - 20 kHz, with a precision (AH 2700) bridge, and extended up to 2 MHz (with an option for DC-bias ±40 V), with an LCR meter (Agilent E4980A).
- continuous flow cryostat (T= 1.8-320 K)
- superconducting magnet (H= 0-7 Tesla)

The nanochemistry facility entails exploitation of elaborate colloidal chemistry approaches (ambient and high-temperature) to harness nanoscale size and shape-guiding mechanisms that afford various kinds of functional nanocrystals (single-phase, core@shell, anisotropic, hybrid particles) with tunable response (semiconducting, metallic, magnetic etc). Multidimensional nanostructures, such as cluster-like nanoarchitectures or periodic nanoparticle superlattices could also be realized by exploiting our know-how on directed assembly methods in liquid media.
Applications:
The aim is to provide a user-oriented platform for cost-efficient, easily scaled-up fabrication of novel inorganic nanoparticles, as well as their complete understanding that facilitates their use in diverse and interdisciplinary applications, from data storage and electronics to catalysis and biomedical imaging/therapy.
Basic Tools:
Projects benefit from controlled requirements for nanocrystal growth under anaerobic conditions met by the offered tools (e.g. Schlenk techniques, including digital temperature control growth conditions, Ar-circulating glove-boxes) that are combined with an armory of in-house characterization methods (structural, optical, electrical, dielectric, magnetic etc.).
- Chemical hoods equipped with vacuum-inert gas lines (Schlenk type), Glove-boxes, Centrifuges, Digital temperature controlled heating mantles, Magnetic stirrer hot plates, Incubators, Analytical balances
- Conventional and CCD-assisted stereoscopes, KBr hydraulic press, Glass-blowing propane torch
- Single and two-zone programmable furnaces (up to 1600°C) for vacuum or gas-flow reactions, High-vacuum line with portable programmable furnace for CVT (cf. sublimation & degassing), Thermogravimetric analysis

In everyday life generating and measuring temperature is straightforward, but in the quantum world, which reflects the behaviour of atoms, controlling the temperature is extremely more challenging. At very cold temperatures (cf. -269°C or near zero on the Kelvin temperature scale), as atoms are frozen, we can 'see' unique phenomena that would otherwise be masked by the thermal motion of atoms. As, complex materials are more likely to uncover their quantum properties at cold sample environments, low temperature refrigeration is an essential requirement.
Applications:
A tailored-made, small-scale facility that supplies liquid helium (He) for the needs of our variable-temperature physical property measurement equipment. Reaching temperatures of a few degrees Kelvin that is super cold with respect to ambient, employs artificial means, resting on a pumped helium system, built around a digitally controlled pulsed tube cryorefrigerator. With this technology, warm helium gas comes in contact with the Cold Head, where its thermal energy is absorbed into the 4 K (-269°C) heat exchanger. The process reduces the He-gas temperature, increasing its density, dropping it lower inside a condensing chamber; until it contacts the 4 K surface, where it condenses.The facility recycles the valuable helium gas that boils off from the liquid He dewars of the SQUID magentometer and the magento-electric workstation cryostat operating in our Lab.
Specifications:
The CRYOMECH PT410 re-liquefier is designed to recondense the boil off from liquid helium dewar/cryostats. As the boil off rates may vary depending on the type of temperature-dependent experiments being carried out, we have developed a peripheral medium-pressure vessel assembly, where the excess He gas is stored for future use. This gas is prone to contamination from impurities, like O2 and N2 species, which can reduce the efficiency of the re-liquefier. To this extent a custom-made, digitally-controlled He-gas purification system has been engineered to remove the impurities from the He-gas stream by means of chemical adsorption techniques. All in all, the facility is designed to return the liquid helium to the original dewar/cryostat, establishing a closed He loop, with an average liquefication rate of about 10 lt/day.

Simultaneous Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) based on a computer-controlled SDT Q600 TA-instruments apparatus.
Applications:
SDT Q600, is an analysis system capable of performing DSC and TGA at the same time, and as such it removes experimental and sampling variables in data analysis. The information provided differentiates endothermic and exothermic events, which have no associated weight change (e.g. melting and crystallization or even long-range magnetic order) from those which involve a weight change (e.g. degradation).
Specifications:
The SDT measures the heat flow and weight changes associated with transitions and reactions in materials, from ambient temperature and up to 1500°C. The system entails a physical property sensor (cf. thermocouple, balance), a controlled atmosphere (e.g. noble gas, O2/air) furnace and a temperature programmer, all interfaced to a computer, allowing for a bi-modal operation, namely:
TGA characterizes any material that exhibits weight loss or phase changes as a result of decomposition, dehydration, and oxidation. Two modes are commonly used for investigating thermal stability behavior in controlled atmospheres: (a) dynamic, in which the temperature is increased at a linear rate, and (b) isothermal, in which the temperature is kept constant.
DSC is acomplissed by employing a single heat source and two symmetrically located and identical sample platforms at the end of two parallel beams. Thermocouples, welded at the center of the sample platforms, measure the differential heat flow to the sample and reference as both are heated at a uniform rate by the furnace. Sample temperature is also monitored directly by the thermocouple in the sample platform. With proper calibration, the heat flow associated with endothermic and exothermic transitions in materials can be measured to a high degree of accuracy and precision (+ 2%). Without calibration, the heat flow results obtained are qualitative (DTA).
Sample-containing cup sizes
-
alumina ceramic 40 µL, 90 µL (recommended for DSC-TGA studies)
-
platinum 40 µL, 110 µL (recommended for TGA-DTA studies)

A preparative solid state chemistry laboratory is set up, where various synthetic techniques including sol-gel and redox precipitation processes, in addition to conventional solid-state high-temperature methods, are been implemented.
The materials straddle to a portfolio of transition metal oxides, mixed-metal chalcogenides, and all the way to hybrid perovksites.
In addition, intermetallic compounds can be grown with an arc-melting furnace (>2000 oC) equipped with a water-cooled copper hearth. The system is easily purged (vacuum & Ar-gas) allowing specimens of metal ingots to be rapidly formed with good purity.
Basic Tools:
- Glove-boxes for Air- and Moisture- Sensitive Compounds
- High Vacuum (P<10-4 mbar) or Helium-Flow Glass Line for Medium-Temperature (<1100 oC) for Solid-State Syntheses.
- High-Temperature Programmable Electric Furnaces (<1600 oC) for Solid-State Reactions.
- Chemical Vapor Transport Reactions (<1000 oC)
- Intercalation Reactions ("Soft Syntheses" at 40-80 oC).
- Solvo-/ Hydro- thermal Reactions (Teflon-lined Autoclaves: 23 mL, 250 oC, 1800 psi).
- Thermal Evaporator (Thick Film Growth: ~100 μm ).
Where necessary samples are flame sealed in evacuated glass or silica ampules and annealed at the required temperatures.
Distinctions
U.S. Grant Award (Fulbright Greece)
Fulbright Award (FORTH Research Highlights)
3rd Applied Research & Innovation Competition («Η ΕΛΛΑΔΑ ΚΑΙΝΟΤΟΜΕΙ»)
Chaire TOTAL de la Fondation Balard (Pôle Chimie Balard)
Outreach
Disorder in Magnetic Nanocrystals May Improve Cancer Treatment (BNL Feature Story)
Alexandros Lappas 2016-2017 Fulbright Visting Scholar (Greek Alumni Speak - Highlights Achivements)
Frustration in Two-dimensions (ESRF Scientific Highlights)
On-line lectures/ interviews
Tweaking Nanoscale Magnetism (E-MRS 2021 Spring Meeting, Symp-S - Invited Talk; credit IESL-FORTH)
Nanomaterials for Energy & Health Applications (ERT TV 'O3' - Interview; Greek language)
In the Battle Against Cancer (TV CRETA 'De Facto' - Interview; Greek language)
Broader Readership
Nanoscale Particle Organization & Photonics (credit IESL-FORTH)
Nanocrystals: Good Things in Small Packages (credit IESL-FORTH)
Lab website (early times)
Functional Nanocrystals and Quantum Magnetism Lab (External Website)