News
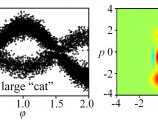
In 2025, the Journal of Physics B: Atomic, Molecular and Optical Physics (IOP Publishing) highlighted a selection of outstanding works that shaped atomic and molecular physics during the year. Among these was a review article led by researchers from the Foundation for Research and Technology-Hellas (FORTH), Institute of Electronic Structure and Laser (IESL), underscoring the institute’s leading role in research on strong-field and quantum light–matter interactions.
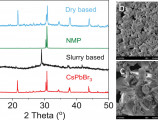
The open access research paper entitled "Processing and Compositional Effects on the Stability of All-Inorganic Metal Halide Perovskite Anodes: A Comparative Study of Dry- vs. Slurry-Fabricated Electrodes," resulting from a collaboration between the Institute of Electronic Structure and Laser (IESL-FORTH), the Democritus University of Thrace (DUTH), and the University of Crete, has been published in the prestigious journal Advanced Materials Technologies (Wiley).
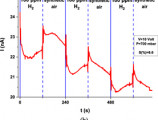
The peer-reviewed research paper entitled “Self-powered, flexible and room temperature operated solution processed hybrid metal halide p-type sensing element for efficient hydrogen detection”, resulting from a collaboration between the Institute of Electronic Structure and Laser (IESL–FORTH), the University of Crete, and the Hellenic Mediterranean University (HMU), has been selected for inclusion in a curated collection of JPhys Materials (IOP Publishing).